Pirfenidone*
-
API Product :
Pirfenidone*
-
CEP :
-
-
WCC :
?
-
Therapeutic Use :
Lipid-regulating/anti-atheroma preparations
-
Originator :
Auspex Pharmaceuticals
-
CAS No. :
53179-13-8
-
Trade Name. :
Esbriet, Pirespa, Etuary
-
Molecular Weight :
185.22 g/mol
-
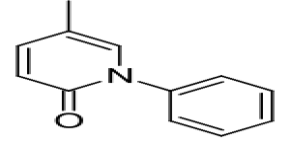
Molecular Formula :
C12H11NO
Application
Pirfenidone is used to treat a certain lung disease called idiopathic pulmonary fibrosis (IPF). This disease causes the lungs to get scarred and become stiff, making it hard to breathe. Pirfenidone may help slow down the worsening of your IPF.
General Description
Pirfenidone is a medication used for the treatment of idiopathic pulmonary fibrosis. It works by reducing lung fibrosis through downregulation of the production of growth factors and procollagens I and II. It was developed by several companies worldwide, including the original patent holder, Marnac,[2] InterMune Inc. (now part of Roche), Shionogi Ltd., and GNI Group Ltd. In 2008, it was first approved in Japan for the treatment of patients with idiopathic pulmonary fibrosis after clinical trials, under the trade name of Pirespa by Shionogi. In October 2010, the Indian Company Cipla launched it as Pirfenex. In 2011, it was approved for use in Europe for idiopathic pulmonary fibrosis under the trade name Esbriet;[3] it was approved in Canada in 2012 under the trade name Esbriet; and was approved in the United States in October 2014 under the same name. In September 2011, the Chinese State Food and Drug Administration provided GNI Group Ltd with new drug approval of pirfenidone in China,[4] and later manufacture approval in 2013 under the trade name of Etuary.