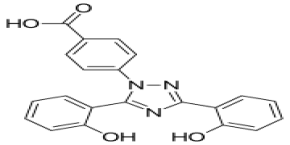
Deferasirox*
-
API Product :
Deferasirox*
-
CEP :
-
-
WCC :
?
-
Therapeutic Use :
Vitamins; All Other Therapeutic Products; Antivirals for systemic use; Systemic agents for fungal infections
-
Originator :
NOVARTIS RX
-
CAS No. :
201530-41-8
-
Trade Name. :
EXJADE
-
Molecular Weight :
373.362 g/mol
-
Molecular Formula :
C21H15N3O4
Application
Deferasirox is used to treat iron overload caused by blood transfusions in adults and children at least 2 years old. Deferasirox is also used to treat chronic iron overload syndrome caused by a genetic blood disorder in adults and children who are at least 10 years old.
General Description
Deferasirox (marketed as Exjade,[2] Desirox, Defrijet, Desifer, Rasiroxpine and Jadenu) is an oral iron chelator. Its main use is to reduce chronic iron overloadin patients who are receiving long-term blood transfusions for conditions such as beta-thalassemia and other chronic anemias.[3][4] It is the first oral medication approved in the USA for this purpose.[5] It was approved by the United States Food and Drug Administration (FDA) in November 2005.[3][5] According to FDA (May 2007), renal failure and cytopenias have been reported in patients receiving deferasirox oral suspension tablets. It is approved in the European Union by the European Medicines Agency (EMA) for children 6 years and older for chronic iron overload from repeated blood transfusions.